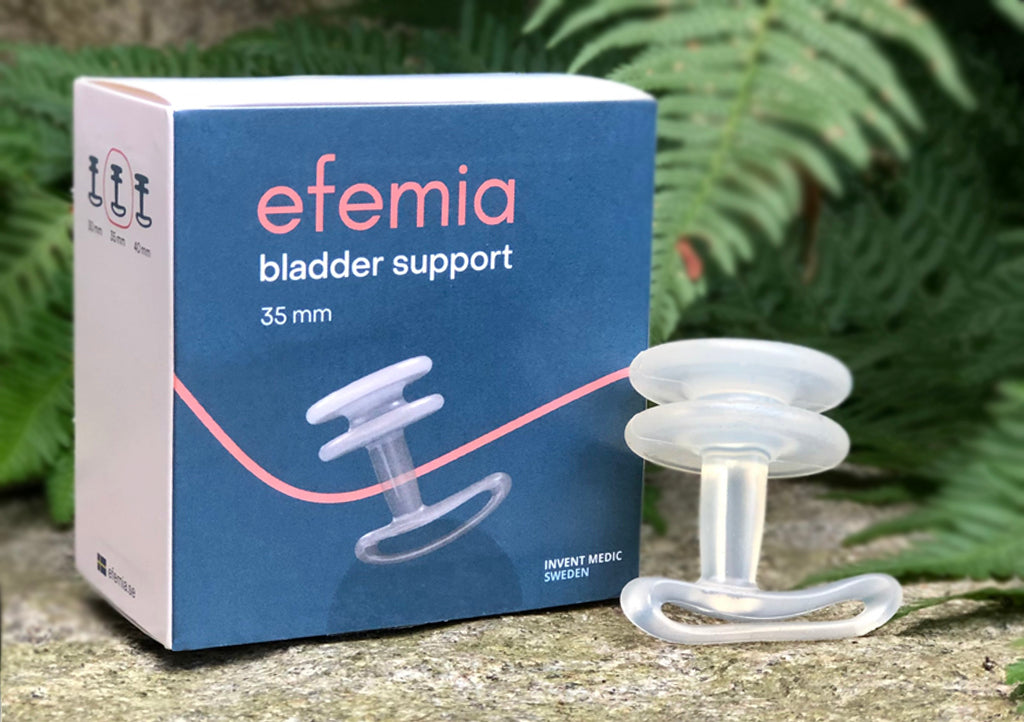
Efemia Development & Design
The development and design behind Efemia Bladder Support is based on the integral theory, the same scientific theory as the most common surgical methods for stress urinary incontinence, TVT...but Efemia excludes the need for surgery, and subsequent risks involved with TVT mesh implantation.
Efemia is designed to support the urethra through the vaginal wall by the two support rings. Without the need for any surgery. The rings support the "urethral knee" located at the mid-urethra when the urethra and bladder neck drop at elevated abdominal pressure.
The two support rings of Efemia have the same distance apart as the width of the TVT mid-urethral mesh sling. The length of the Bladder Support stem is designed so that the support rings are situated in the same position as the TVT sling. The handle holds the device at the right position. The shape of the rings ensures that it is effective, regardless of the direction in which the handle points, thus minimising user errors.
Material
- 100% medical grade pure silicone.
- Soft and flexible, but sufficiently stable to support the urethra during periods of increased abdominal pressure.
- The material is durable and resilient so it can withstand the vaginal environment.
- Contains no heavy materials, flame retardants, phthalates, bisphenols (used to make polymers and resins, which are then used to make plastic materials), fluorinated compound, PFAS (synthetic chemicals), colourants, antibacterial substances, latex or animal proteins.
- The material is tested for biocompatibility and conforms to the standards for medical devices, ISO10993-1:2018 and USP class IV